Living cells have a fascinating ability to generate complex and dynamic internal structures. Nowhere is this property more evident than during mitosis and cytokinesis: in a very short time (often of the order of a few minutes) cells alter their shape, duplicate and partition their internal components, and divide into two apparently identical halves. These dramatic morphological changes need to be carefully coordinated with each other in space and time. To learn more about the principles underlying this coordination, we focus on the events at the end of the cell cycle: chromosome segregation and cytokinesis, in the yeast Saccharomyces cerevisiae. Regulatory systems identified in yeast are then validated in animal cells (such as Drosophila), to ensure that our key findings are relevant for the fidelity of mitosis and genetic stability in multicellular organisms.
Accurate partitioning of the genetic material during cell division is critical for the maintenance of genomic stability, both within organisms and across generations. Defects in chromosome segregation produce aneuploidy – an unequal distribution of chromosomes between daughter cells –, which can cause developmental defects and is a hallmark of cancer. To ensure error-free transmission of chromosomes, feedback control systems, known as checkpoints, verify that processes at each stage of the cycle have been completed, before allowing progression into the next stage. For instance, the spindle assembly checkpoint prevents initiation of anaphase until chromosomes attach properly to the spindle. Until recently, little was known about mechanisms protecting cells against errors in chromosome segregation after anaphase has begun. Yet late segregation errors, such as lagging chromosomes and anaphase bridges, are not rare in normal conditions, and are frequent in tumours. Late-segregating DNA is thus exposed to potential damage by the cell division machinery. How do cells react to such a threat?
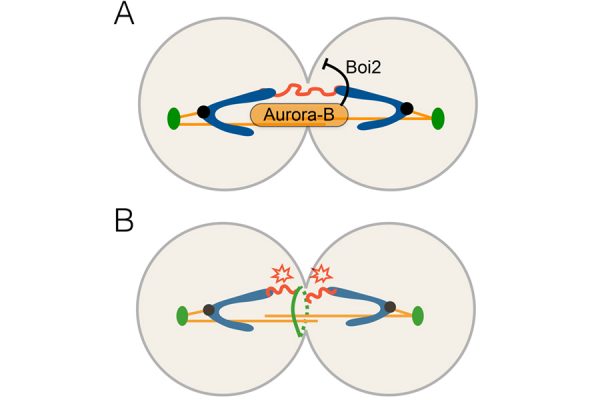
Given the potentially damaging consequences of these chromosome segregation defects, cells have developed mechanisms to protect chromatin bridges from damage during cytokinesis. We previously identified the “NoCut” checkpoint, which inhibits the last step of cytokinesis in response to anaphase chromatin bridges, in order to prevent DNA damage. NoCut depends on the activity of the kinase Aurora-B, a component of the Chromosome Passenger Complex (CPC) which localizes to spindle midzone microtubules during chromosome segregation. Current evidence suggests that Aurora-B detects the presence of lagging DNA and, in response, inhibits the cleavage of the plasma membrane at the end of cytokinesis – a process termed abscission (Figure 1).
Direct regulators of abscission downstream of Aurora B in budding yeast include the cortical Boi proteins. The mechanism(s) by which these proteins regulate abscission timing remained unclear. This past year, we demonstrated that NoCut-dependent abscission inhibition requires plasma membrane remodelling during cytokinesis by the cortical factor Boi2 (Masgrau, Battola et al., Molecular Biology of the Cell, 2017). We found that Boi2 regulates the function of the exocyst, a conserved protein complex essential for fusion of secretory vesicles with the plasma membrane. This work revealed a novel interplay between the secretory pathway and the regulation of cytokinetic abscission by the NoCut checkpoint. This project was a collaboration with the lab of Toni Gabaldón (Bioinformatics and Genomics).